In an article published this week in the Journal of Biological Chemistry, the Schlieker, Xiong, and Gerstein labs identified a new molecular player in the maintenance and modulation of endoplasmic reticulum (ER) membranes.
The ER is composed of three regions with distinct membrane structures – the nuclear envelope, tubules, and sheets. The membrane structures of each of these regions are necessary for maintaining the unique functions of each region. The authors describe that the proteins involved in shaping ER tubules are well established. However, evidence from depletion studies of known ER sheet-shaping proteins pointed to the existence of additional, unidentified proteins involved in sheet morphology maintenance.
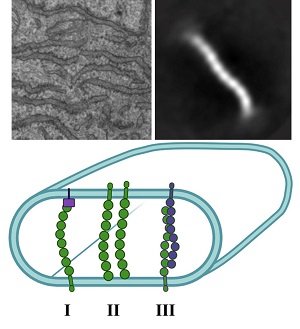
The team began searching for this potential new ER-shaping protein by using a proximity ligation assay. They identified nodal modulator 1 (NOMO1) as an abundant ER-luminal protein, but it had no known ER function. They then characterized the structure and function of NOMO1 to determine whether it plays a role in shaping ER membrane morphology. Depletion and overexpression of NOMO1 revealed that ER morphology was dependent on NOMO1. Structural analysis by electron microscopy further supported the role of NOMO1 in membrane spacing. They found that the protein was rod-shaped, with a length nearly equal to the membrane spacing observed upon overexpression of NOMO1.
This work identifies NOMO1 as a new member of a functional network of ER-shaping proteins, and introduces a new model for understanding how the ER membrane can dynamically adjust while maintaining overall structure.
The full article can be found here: https://www.jbc.org/article/S0021-9258(21)00737-7/fulltext
By Melanie Reschke