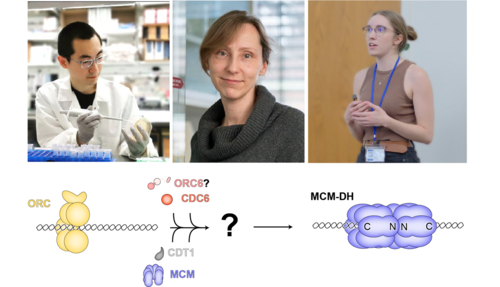
Accurate and timely DNA replication is the cornerstone of life, ensuring that genetic information is faithfully passed to the next generation. In eukaryotic cells, this process begins with the licensing of replication origins, where the MCM2-7 protein complex is loaded onto DNA. During DNA replication, MCM2-7 resides at the heart of the DNA replication machinery and serves as the molecular motor that unwinds DNA. Successful origin licensing requires tight association of two loaded MCM2-7 complexes into MCM double hexamers, establishing the foundation for bidirectional replication. While decades of research in budding yeast have illuminated much of this process, the mystery of how it unfolds in human cells has remained an intriguing puzzle —until now.
A recent study from the Bleichert Lab, published in Nature, has uncovered new details about how the human MCM double hexamer is loaded onto DNA. Graduate students Ran Yang and Olivia Hunker in MB&B employed biochemical reconstitution and electron microscopy to investigate the unique features of human origin licensing. Their work revealed an unexpected divergence between yeast and human MCM loading mechanisms, particularly with respect to the role of the origin recognition complex (ORC) that recruits and loads MCM2-7 onto origins.
Notably, the study revealed that the ORC6 subunit, essential for origin licensing in yeast, is dispensable for this process in humans. Moreover, ORC forms intermediate complexes with MCM that are architecturally distinct from those in yeast. To understand the function of these unique complexes, the Bleichert Lab utilized electron microscopy to structurally characterize intermediates in MCM double hexamer formation, finding that closed-ring single hexamer intermediates can mature into double hexamers through multiple pathways. In two of these pathways, ORC directs the recruitment of the second MCM hexamer through the establishment of two different MCM-ORC complexes. A surprising discovery was the natural propensity of human MCM hexamers to self-dimerize, facilitating an alternative third pathway where double hexamers form from independently loaded single hexamers.
These combined insights culminated in a model proposing several distinct mechanisms for loading the human MCM helicase onto replication origins. This surprising flexibility in human MCM loading pathways suggests that different replication origins in human cells could be licensed through distinct mechanisms, which may increase their resilience against replication stress.
The in vitro reconstitution of human MCM loading represents a breakthrough, providing a long-awaited platform to explore the intricacies of human DNA replication initiation through biochemistry, biophysics, and structural biology. This system also paves the way for reconstructing the entire replication initiation pathway in humans, which will allow researchers to address fundamental questions about genome stability and the mechanisms that ensure accurate DNA replication.
Read more here: https://doi.org/10.1038/s41586-024-08237-8