The Neugebauer Group recently discovered that multimerization by coilin and interactions with the protein Nopp140 guide formation of biomolecular condensates in the nucleus. Titled “The coilin N-terminus mediates multivalent interactions between coilin and Nopp140 to form and maintain Cajal bodies,” their publication in Nature Communications is led by first authors Edward Courchaine, Sara Gelles-Watnick, and Martin Machyna.
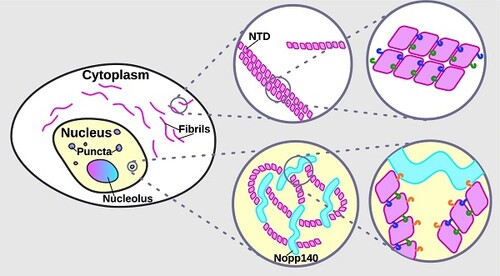
Cajal bodies (CBs) are nuclear membraneless organelles that serve as sites of modification and assembly of several Uridine-rich small nuclear ribonucleoproteins (U snRNPs) involved in splicing. Though known to help concentrate components involved in nuclear processes to CBs, the mechanism by which coilin drives this process has remained unknown. The new findings from Courchaine et al. shed light on this process, which involves “the unexpected capacity of coilin’s N-terminal domain (NTD) to form extensive fibrils in the cytoplasm and discrete nuclear puncta in vivo.”
Efforts from the group revealed that molecular interactions between the N-terminal domain of multiple coilin proteins form fibrils and interact with Nopp140 to create rounded puncta. Interestingly, the group found that Nopp140 can participate in condensation to further drive Cajal Body formation.
The initial coilin- and Nopp140-driven assembly of the spherical membraneless structure then grows in increasingly complexity to form mature CBs. According to the publication, the data “reveal specific multivalent interactions of the coilin NTD that shed new light on CB assembly, provoking an evaluation of the role of biomolecular condensation in this process.”
Other authors that contributed to these findings include Korinna Straube, Sarah Sauyet, and Jade Enright. More details on the study can be found in Nature.
By Brigitte Naughton