The Chung Lab (Jean-Ju Chung, PhD, Cellular & Molecular Physiology and the Zhang Lab (Kai ‘Jack’ Zhang, Molecular Biophysics & Biochemistry) published a collaborative research article on male infertility in eLife. The manuscript, entitled “LRRC23 truncation impairs radial spoke 3 head assembly and sperm motility underlying male infertility,” is an excellent example of how joint efforts between labs with various expertise can work together to study human disease. By combining their expertise through functional, pathological, and structural studies, the groups were able to make important findings regarding the role of LRRC23 (Leucine-rich repeat containing 23 protein) in the radial spokes (RS) within the axonemal structure of sperm flagella. The Chung lab led this project with the functional studies, and co-first author Pengxin Chai (MB&B PhD candidate) of the Zhang lab performed the Cryo-ET work and structural analysis.
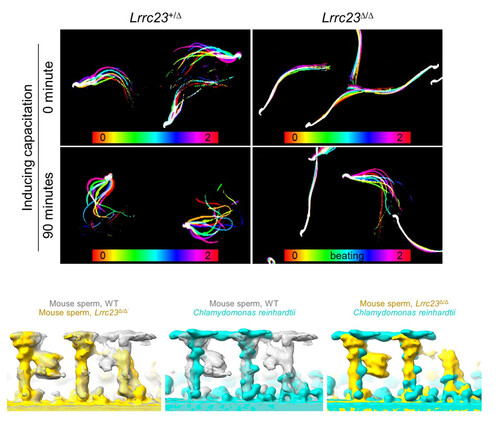
Radial spokes are T-shaped multiprotein complexes that play a key role in regulating the motility of cilia and flagella. In most organisms, the radial spokes are arranged in specific pattern, and each head is named respective to their sequential arrangement along the axoneme as RS1, RS2, and RS3. LRRC23 is a protein that is a critical component of RS3 and has been shown to play an important role in male infertility when mutated. By characterizing a C-terminally truncated LRRC23 derived from an asthenozoospermia patient with a loss-of-function splicing variant in LRRC23. Mutant mouse models were made to mimic the human LRRC23 variant, and the mouse model was consistent with the human patients, showing that this variant causes impaired sperm motility. The mouse model also helped determine that the truncated LRRC23 failed to localize correctly in the sperm tail and is correlated with defective RS3 head assembly.
Previously believed to be an ortholog of the RS stalk protein RSP15, LRRC23 is now presented in a different light by the recent study from the Chung and Zhang labs, proposing an alternative model that challenges established views. Since it was thought LRRC23 was part of the RS stalk assembly, they tested the hLRRC23 and hLRRC23 mutant’s ability to interact with RS stalk or RS head proteins. Interestingly, wild-type hLRRC23 only interacted with RSPH3, an RS head protein, but none of the RS stalk proteins. Through BLAST and AlphaFold-predicted structures, they determine LRRC34 is the ortholog of the RS stalk protein RSP15, not LRRC23.
To better understand how LRRC23 is incorporated into RS3, the team performed cryo-ET to visualize the substructural changes between WT and the Lrrc23∆/∆ sperm axoneme. They observed that the absence of LRRC23 doesn’t alter the structural features of RS1 or RS2, but the densities corresponding with RS3 are much weaker in Lrrc23∆/∆. To better visualize the impacts of LRRC23 on RS3, they performed sub-tomogram averaging (STA) of the axonemal doublet to capture the RSs in more detail. The 3D maps revealed that in the Lrrc23∆/∆ sperm, the entire head region of RS3 is missing. The cryo-ET and STA analysis were also able to show that LRRC23 is essential for mammalian sperm-specific bridge structure between RS2 and RS3 which they speculate could be important for asymmetric flagellar motility that is unique to mammalian sperm hyperactivation.
This article from the Chung and Zhang labs is a great example of a profound discovery that can be made through collaborations approaching the same questions using complementary structural and functional techniques, perspectives, and expertise. Congratulations to both labs for their new publication, and read the full article here.
Jake Thrasher