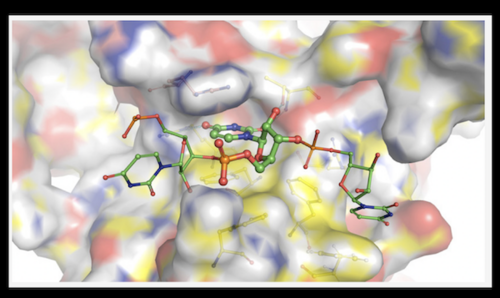
Jimin Wang and colleagues recently characterized the Human Polypyrimidine Splicing Factor (PSF/SFPQ), an important tumor suppressor protein and multifunctional protein that binds RNA to regulate gene expression of protooncogenes, among other critical functions. PSF is also known to bind to RNA of certain viruses, including SARS-CoV-2. However, the method by which PSF recognizes RNA is not known. In this paper, the authors use x-ray crystallography to report multiple structures of biologically active PSF. While PSF is a highly studied protein and structures of this constructs has been reported previously, this paper provides novel insight by solving a PSF-nucleic acid complex. This complex was shown to form a dimer, allowing for two specific induced fit binding pockets to recognize its RNA substrate. Furthermore, the dimer appeared to have distinct conformations in the apo-form and the complexed form, the first with low affinity for RNA and the second with high affinity. The authors believe the structures inform a new regulatory mechanism of PSF that is likely involved in oncogenesis and other human diseases.
All authors are affiliated with the MB&B department, including Jimin Wang, Aristidis Sachpatzidis, Thomas D. Christian, Ivan B. Lomakin, Alan Garen, and William H. Konigsberg. The paper can be found in the Biochemistry journal of ACS publications.
By Shravani Balaji