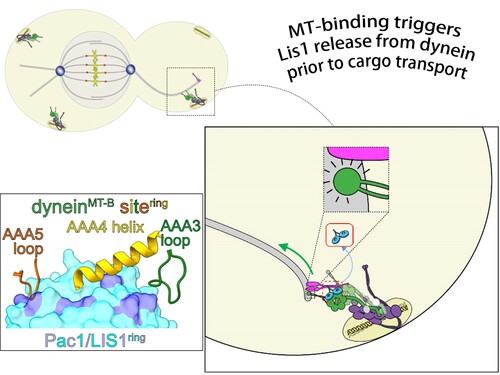
Dynein, a motor protein, acts much like a vehicle in our cells by transporting essential cargo along microtubule (MT) ‘highways.’ Disruption of dynein’s function or misregulation of its cofactors can lead to a variety of human diseases, making the understanding of its regulation crucial. The binding and unbinding of one such regulator, LIS1, is required for cytoplasmic dynein-1 activity. How these steps are coordinated to ensure proper initiation of cargo transport, however, remained unclear.
Now, cryo-EM structures reported by the authors describe in temporal and spatial detail how LIS1 binds and releases from dynein to enable its proper function. High-resolution structures of dynein-1 in both the absence and presence of LIS1 showed limited conformational changes to dynein upon LIS1 binding, suggesting that it is the dynein-MT binding state that determines dynein-LIS1 affinity. The researchers concluded that this process is triggered by conformational changes in dynein upon binding or release from microtubules.
To further understand the binding mechanism between LIS1 and dynein, the team employed protein engineering technologies to lock both yeast and human dynein in two distinctive states – either bound (MT-B) or unbound (MT-U) to microtubules. They found that while the MT-B mutant exhibited low affinity for LIS1, MT-U exhibited exceptionally high affinity that resulted in its retention on MT plus-ends. Despite dynein functioning as a dimer, the authors also demonstrated that that a single evolutionarily conserved motor domain is sufficient for dynein binding to LIS1.
These findings provide a crucial understanding of the conformational changes underlying dynein’s interactions with microtubules and LIS1, and in turn the molecular mechanisms underlying dynein function and regulation. The collaborative work toward an improved understanding of dynein-mediated intracellular transport mechanisms could pave the way for new treatments for diseases caused by dynein dysfunction.
Co-first authors include William D. Ton, a Ph.D. student in the Markus Lab, Pengxin Chai, an MB&B Ph.D. student in the Zhang lab, and Dr. Yue Wang, an associate research scientist in the Zhang lab. The Markus Lab performed the functional studies, and the Zhang lab determined the cryo-EM structures. Yale cryo-EM facility and the Brookhaven National Laboratory provided technical support. The full findings can be found in Nature Structural and Molecular Biology: https://www.nature.com/articles/s41594-023-01010-x.
By Brigitte Naughton